Research
Lipid flux and cellular self-organization
Artwork by David S. Goodsell, RCSB Protein Data Bank (CC BY 4.0).
Lipids are fundamental building blocks of cellular life. They serve not only as energy stores, but as dynamic regulators of membrane identity, organelle biogenesis, signaling, and metabolic adaptation. Yet many core principles remain unknown.
How do cells decide when to store lipids versus mobilize or secrete them?
How do membranes remodel to generate new organelles?
How do lipid environments encode information that guides protein activity?
Although lipid imbalances contribute to major human diseases, including fatty liver, obesity, cardiovascular disease, and neurodegeneration, the molecular logic organizing lipid flux inside cells is still largely unresolved.
In-cell structural biology of membrane remodeling
We combine advanced cell biology with cutting-edge cryo-correlative light and electron tomography (cryo-CLEM ET) to visualize lipid-driven remodeling events directly inside intact cells. In-cell cryo-ET enables pristine preservation of membrane architecture and can allow for near-atomistic resolution of macromolecular machinery in the native cellular environment. Our recent work using genetically encoded nanoparticles (GEMs) now enables nanometer-precision localization of proteins in cryo-ET, opening the door to capturing rare and transient membrane intermediates with exceptional clarity. In parallel, recent advances in cryo-lift-out methodology now enable larger samples, such as high-pressure-frozen tissues, to be examined with cryo-ET.
By integrating in-cell cryo-ET and advanced cell biological tools, our goal is to illuminate how organelles emerge, transform, and coordinate metabolic decisions across scales—from single lipids to whole-cell and tissue-level architecture.
Research themes
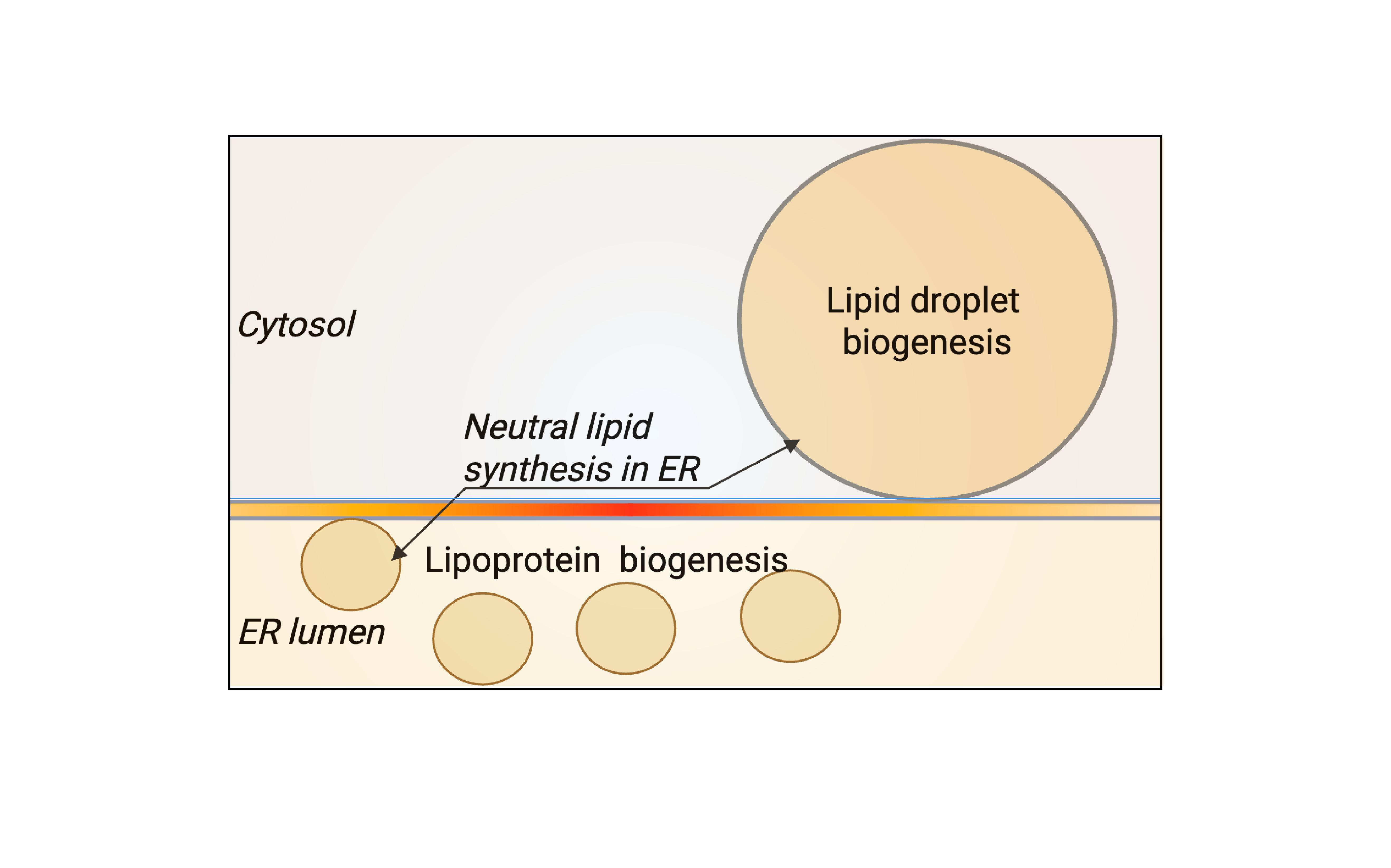
1. Nanoscale architecture of LD and VLDL formation
Lipid droplets (LDs) and very-low-density lipoproteins (VLDLs) originate from the same ER membrane but drive opposing metabolic fates: lipid storage versus secretion. Dysregulation in either pathway leads to disease: excessive storage of lipids in LDs drives fatty liver disease, while excess secretion via VLDLs promotes dyslipidaemia and atherosclerosis. We are interested in how ER nanodomains bias lipid flux toward one pathway or the other.
Open questions include:
- What architectural features define LD- versus VLDL-forming ER regions?
- How do neutral-lipid clusters transition from embedded lenses to mature LDs?
- How is substrate competition between LD and VLDL pathways balanced?
2. Cryo-CLEM tools for visualizing lipid environments
A central theme of the lab is to observe cellular machinery directly inside intact cells. We combine cryo-CLEM, cryo-FIB lamella preparation, cryo-ET with subtomogram analysis, targeted fluorescent reporters, and controlled lipid-flux perturbations to follow how organelles remodel at nanometer resolution.
A key frontier for us is understanding the local lipid environments that shape these processes. While current structural methods excel at visualizing proteins, pinpointing specific lipid chemistries in situ remains technically challenging. We are exploring cryo-CLEM strategies, lipid-sensitive probes, and correlative workflows that may help place lipid context more accurately into cryo-ET data.
By integrating these approaches, we aim to connect membrane organization, protein function, and metabolic state within a shared structural framework.
Open questions include:
- How can we directly label distinct lipid species in native membranes?
- Can we track dynamic lipid reorganization during organelle remodeling?
- What molecular features define “lipid hotspots” that drive flux?

3. Seipin regulators and the control of lipid flux
Seipin is a central organizer of lipid droplet biogenesis, yet its regulation and interaction network remain incompletely understood. We are exploring what factors shape seipin’s activity.
Open questions include:
- How do seipin-associated proteins tune lipid storage decisions?
- What conformational states correspond to active versus inactive LD assembly?
- How are regulatory cues integrated at nanoscale ER subdomains?
4. Neutral-lipid homeostasis and ferroptosis
Ferroptosis—an iron-dependent cell death pathway driven by lipid peroxidation— strongly interacts with lipid storage and metabolic state. We are interested in how neutral-lipid pools, LDs, and ER membranes influence susceptibility to ferroptotic damage.
Open questions include:
- Where does membrane damage originate at the nanoscale?
- Do LDs buffer, propagate, or prevent lipid peroxidation?
- How do lipid flux states shape cell fate during stress?